
Rabies Antiserum
Product Specification
400IU(2ml)/vial,5vials/box
Product introduction
Rabies, also known as aquaphobia, isan acute infectious disease of central nervous system commonly suffered by both humans and animals (as caused by rabies virus). It is generally seen in carnivores such as canines, wolves, and cats. Human beings can be infected after being bitten by rabid animals. The domestic dog population is the most common reservoir of the virus causing more than 95% of human cases. Clinical manifestation is anemophobia, hydrophobia, pharyngismus, progessive paralysis, etc., with a clinical mortality rate as high as almost 100%.
Presently, there is no effective treatment for rabies. Therefore, the key to control this disease lies in prevention, with measures generally divided into three categories:
1. Control of animal infectious sources
2. Pre-exposure immune (Preventive vaccine innoculation)
3. Post-exposure immune (Preventive treatment vaccine and rabies antiserum innoculation).
Rabies Antiserum, produced by Shanghai Serum Bio-technology Co., Ltd., is the liquid form of anti-rabies immunoglobulin made from equine plasma obtained from horses that was immunized with rabies vaccine. It is used in the prevention of rabies in combination with rabies vaccine innoculation. The current generation of product is made by state-of-the-art, cutting edge technology with the finest class quality in the world, which, as a result, greatly improves the safety for clinical administration.
Advantages of the Product
· High Purity: Compared with traditional Rabies Antiserum, the impurities reduced sharply, so that the side-effect incidence drops dramatically;
· High efficiency: The active content of anti-rabies virus(F(ab’)2)reaches the international standard;
· Safety: Avoiding the potential risk of human blood transmitted pathogens such as hepatitis, AIDS, etc.
· Economy: Covered by the State Health Insurance, it can reduce the medical burden of patients;
Quality Index
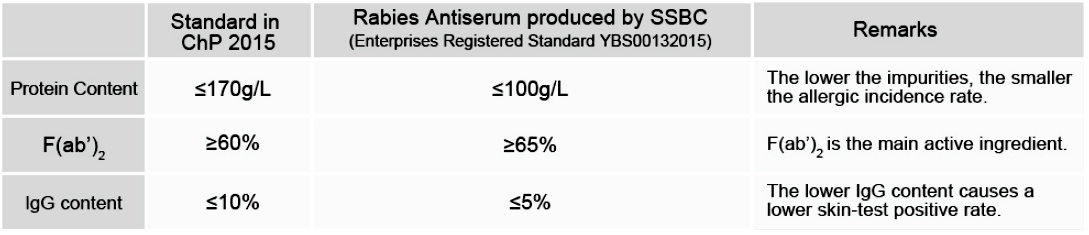
As can be seen, the quality of Rabies Antiserum produced by Shanghai Serum bio-Technology Co., Ltd. is significantly higher than the standards required in “Chinese Pharmacopoeia 2015 (ChP2015).
“Post-Exposure Prevention Guideline for Rabies” by the World Health Organization (WHO)

Category II is described by WHO with the following conditions:
· The person is exposed to the wild animals; or
· The person suffers the immunodeficiency condition, or the person’s head or face is exposed; or
· The person is exposed to the highly suspected rabid animal are recommended to be treated as Category III.
Category III patients will be posed with tremendous risks if they do not receive rabies antiserum passive immunization following the WHO recommendations. As the generation of anti-rabies antibody will occur within 1-2 weeks after injection of rabies vaccine, the patient is susceptive to rabies virus during that period. Thus, it is necessary to inject rabies antiserum after exposure to the suspected rabid animal or patient.
Administration of Rabies Antiserum
· Administration of rabies immunoglobulin should be infiltrated into the depth of the wound and around the wound as much as anatomically feasible.
· Dosage: Equine rabies antiserum should be given in a single dose of 40 IU per kg of body weight.
· Sensitivity to equine rabies antiserum must be determined before it is administered (refer to the product application manual). The physician should be prepared to deal with anaphylactic shock reactions.
· The remaining Rabies Antiserum must be injected in the muscle distant from the vaccine innoculation site. If the wound is large at that area, the antiserum can be diluted with saline and then infiltrated to the wounds by injection.
· It is strongly prohibited to inject the antiserum and vaccine with the same syringe.
The insufficient clinical treatment
Some clinical practitioners only administrate the rabies vaccine without the wound infiltration with antiserum for the Category III patients. This method is insufficient to prevent rabies virus infection.